Medical Devices Vigilance Market Overview:
The medical devices vigilance market encompasses the global industry involved in monitoring, reporting, and analyzing adverse events and incidents related to medical devices. This market includes systems, processes, and services that ensure the safety and efficacy of medical devices throughout their lifecycle, complying with regulatory requirements to protect patient health and improve device performance.
The medical devices vigilance market is experiencing significant growth due to the increasing complexity and usage of medical devices across various healthcare settings. Regulatory bodies worldwide mandate stringent vigilance and post-market surveillance to ensure patient safety and device efficacy, driving the demand for robust vigilance systems. Technological advancements, such as artificial intelligence (AI) and machine learning, have enhanced the ability to monitor and analyze vast amounts of data in real-time, improving the detection and reporting of adverse events.
Additionally, the rising incidence of adverse events, along with heightened awareness among healthcare providers and patients, underscores the need for effective vigilance mechanisms. Challenges such as data privacy concerns, the complexity of regulatory compliance, and the need for interoperability between different systems and databases can hinder market growth.
Read more about this report click here: https://market.us/report/medical-devices-vigilance-market/
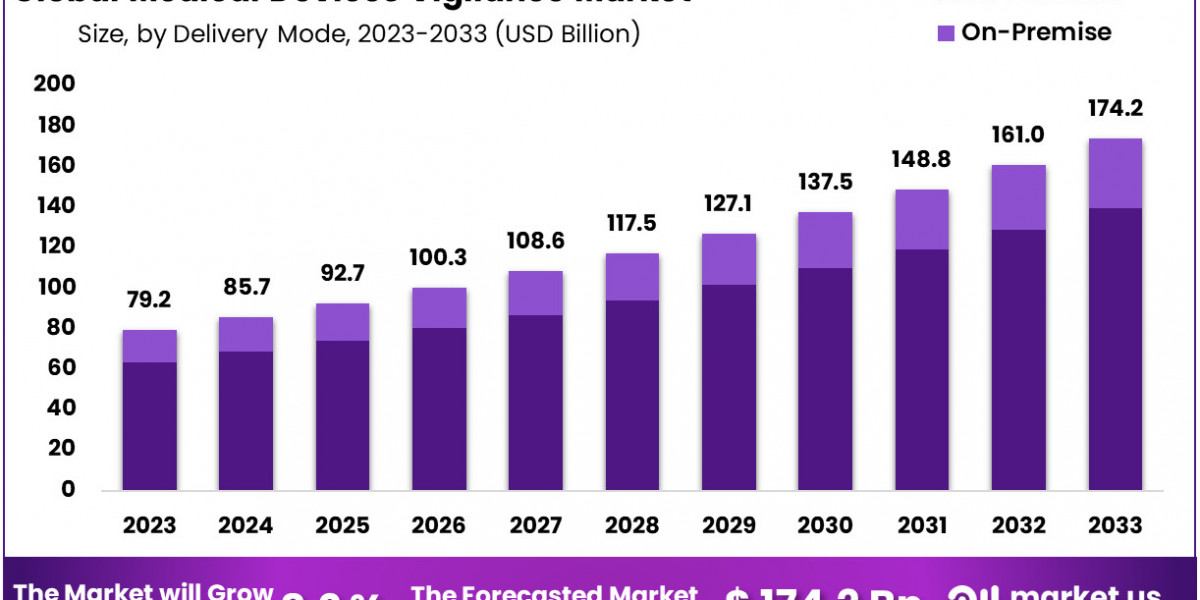
Market Segments
Delivery Mode
- On-Demand
- On-Premise
Application
- Diagnostics
- Therapeutics
- Surgical
- Research
End-User
- Clinical Research Organizations (CROs)
- Business Process Outsourcing (BPO)
- Original Equipment Manufacturers (OEM)
- Other End-Users
Get a Sample Copy of the Report to Know More: https://market.us/report/medical-devices-vigilance-market/request-sample/
Driver:
The primary driver of the medical devices vigilance market is the increasing regulatory requirements for post-market surveillance to ensure patient safety and device efficacy. As medical devices become more complex and widely used, the need for robust monitoring systems to detect and address adverse events grows. Additionally, heightened awareness among healthcare providers and patients about the importance of reporting device-related issues boosts the demand for effective vigilance solutions. Technological advancements, such as AI and machine learning, enhance the capability to analyze large datasets in real-time, further driving the market's growth.
Trend:
A significant trend in the medical devices vigilance market is the integration of advanced technologies, such as AI, machine learning, and big data analytics, to improve the efficiency and accuracy of adverse event detection and reporting. The adoption of digital health technologies, including wearable devices and remote monitoring systems, provides continuous data streams that enhance vigilance activities. There is also a growing emphasis on proactive risk management and predictive analytics, allowing for early identification of potential issues before they escalate into significant problems. This trend towards automation and real-time monitoring is transforming the vigilance landscape.
Restraint:
One major restraint in the medical devices vigilance market is the complexity and variability of regulatory requirements across different regions, which can pose significant challenges for manufacturers in ensuring compliance. Data privacy and security concerns also hinder the seamless sharing and analysis of adverse event data. Additionally, the high cost of implementing and maintaining advanced vigilance systems can be a barrier for smaller companies. Interoperability issues between different healthcare systems and databases further complicate the effective collection and analysis of vigilance data, limiting market growth.
Opportunity:
Opportunities in the medical devices vigilance market lie in the development of advanced analytics tools and platforms that can streamline data collection, analysis, and reporting. Collaboration among industry stakeholders, including regulatory bodies, manufacturers, and healthcare providers, can enhance vigilance practices and improve patient safety. Emerging markets with developing healthcare
If You Have Any Questions About This Report, Please Reach Out to Us https://market.us/report/medical-devices-vigilance-market/#inquiry
Market.us (Powered By Prudour Pvt. Ltd.)
Address: 420 Lexington Avenue, Suite 300,
New York City, NY 10170, United States
Tel: +1 718 618 4351
Email: inquiry@market.u