Report Overview
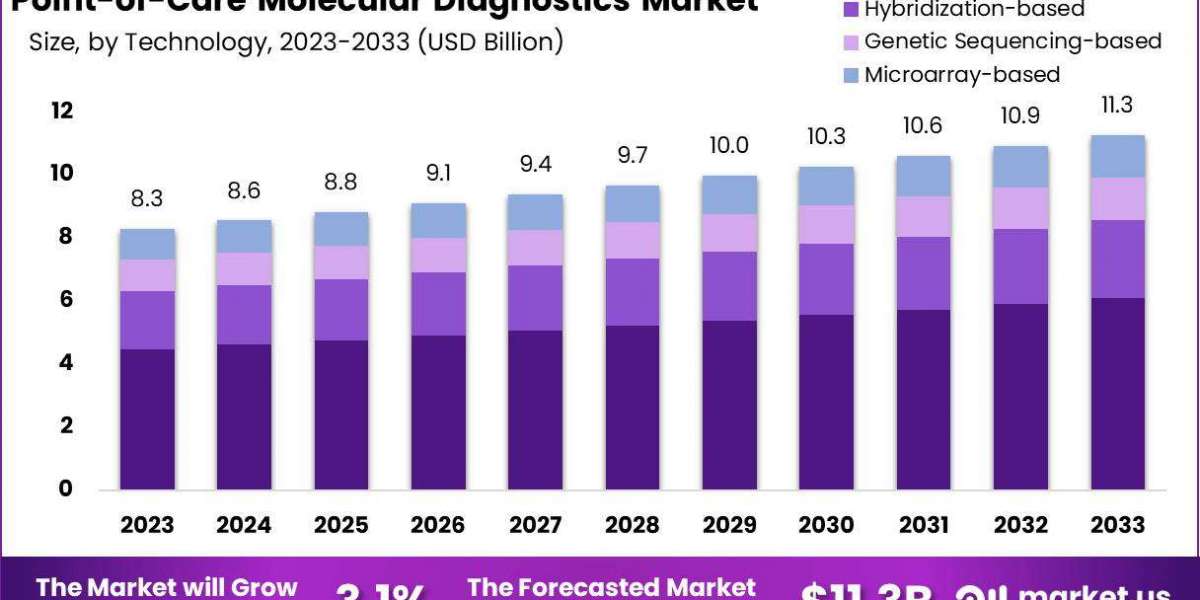
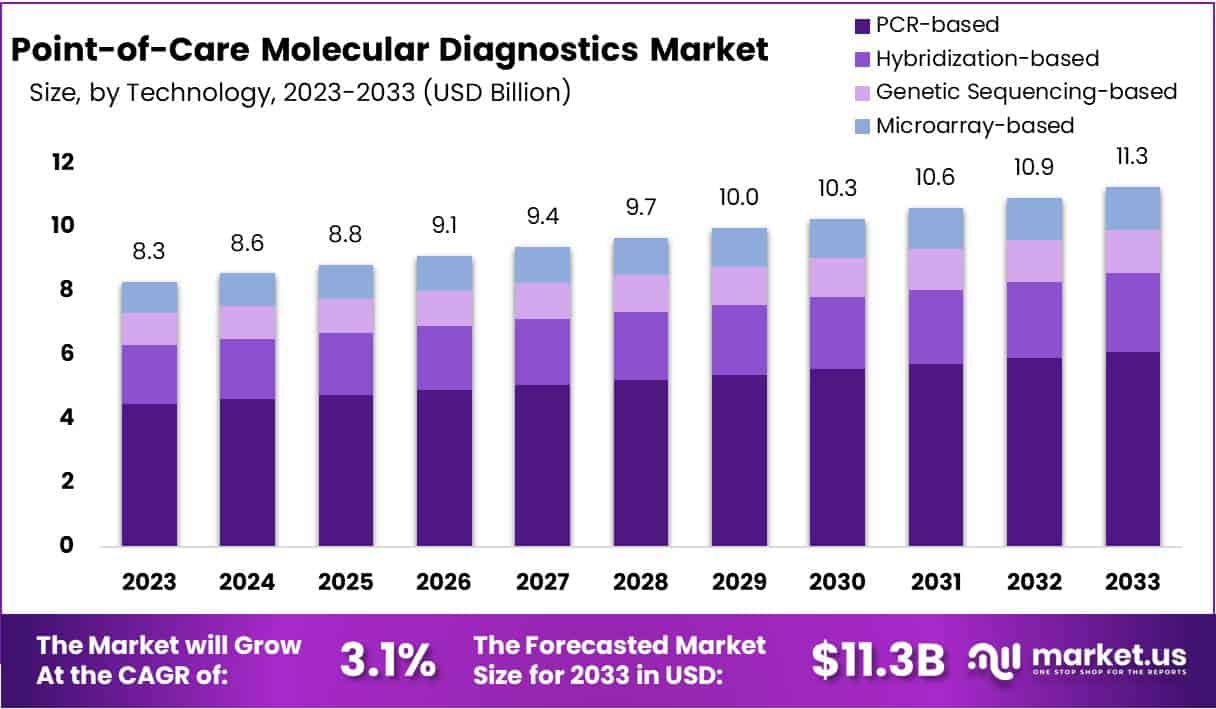
The Global Point-of-Care Molecular Diagnostics Market size is expected to be worth around USD 11.3 Billion by 2033, from USD 8.3 Billion in 2023, growing at a CAGR of 3.1% during the forecast period from 2024 to 2033.
Get a sample copy of the report to know more https://market.us/report/point-of-care-molecular-diagnostics-market/request-sample/
Key Market Segments
Technology
- PCR-based
- Hybridization-based
- Genetic Sequencing-based
- Microarray-based
Application
- Oncology
- Hematology
- Infectious Diseases
- Prenatal Testing
- Other Applications
Test Location
- OTC
- POC
End-use
- Hospitals
- Home-care
- Decentralized Labs
- Research Institutes
- Other End-Uses
Key Regions
- North America (The US, Canada, Mexico)
- Western Europe (Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, Rest of Western Europe)
- Eastern Europe (Russia, Poland, The Czech Republic, Greece, Rest of Eastern Europe)
- APAC (China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, Rest of APAC)
- Latin America (Brazil, Colombia, Chile, Argentina, Costa Rica, Rest of Latin America)
- Middle East & Africa (Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, Rest of MEA)
Market Key Players
- F. Hoffmann-La Roche AG
- Abbott Laboratories
- QIAGEN AV
- Bayer AG
- Nova Biomedical
- Danaher
- Nipro Diagnostics
- Bio-Rad Laboratories Inc.
- Agilent Technologies Inc.
- bioMérieux
- OraSure Technologies
If You Have Any Questions About This Report, Please Reach Out to Us @ https://market.us/report/point-of-care-molecular-diagnostics-market/#inquiry
Drivers
- Rising Demand for Early Disease Detection: The need for timely and accurate disease diagnosis is increasing. Point-of-care molecular diagnostics offer rapid results, driving market growth.
- Technological Advancements: Innovations in molecular diagnostics technology are enhancing the efficiency and accuracy of point-of-care testing. This boosts market expansion.
- Increase in Chronic Diseases: The prevalence of chronic conditions such as diabetes and cardiovascular diseases drives the demand for quick diagnostic solutions.
- Growing Geriatric Population: The aging population is more prone to chronic diseases, leading to a higher demand for point-of-care diagnostics.
- Enhanced Patient Convenience: Patients prefer point-of-care tests for their convenience and speed, which is fueling market growth.
- Cost-Effective Solutions: Point-of-care diagnostics can reduce overall healthcare costs by providing immediate results and reducing the need for further testing.
- Improved Healthcare Infrastructure: Expansion of healthcare infrastructure, especially in remote areas, supports the adoption of point-of-care molecular diagnostics.
Trends
- Integration of AI and Machine Learning: AI and machine learning are being integrated into molecular diagnostics, improving test accuracy and decision-making.
- Increased Focus on Infectious Diseases: There is a growing trend towards developing point-of-care diagnostics for infectious diseases like COVID-19 and influenza.
- Shift Towards Personalized Medicine: Personalized medicine is driving the demand for point-of-care diagnostics tailored to individual patient needs.
- Adoption of Portable Devices: The market is witnessing a rise in portable point-of-care diagnostic devices, making testing more accessible.
- Expanding Applications: Point-of-care molecular diagnostics are expanding beyond traditional uses to include areas like oncology and genetic testing.
- Enhanced Regulatory Approvals: Regulatory bodies are streamlining the approval processes for point-of-care diagnostic devices, encouraging innovation and market entry.
- Growing Consumer Health Awareness: Increased health awareness among consumers is driving demand for accessible and efficient diagnostic solutions.
Opportunities
- Emerging Markets: Expanding healthcare markets in developing countries present significant growth opportunities for point-of-care molecular diagnostics.
- Partnerships and Collaborations: Strategic partnerships between diagnostic companies and healthcare providers can drive market innovation and growth.
- Expansion in Home Diagnostics: The trend towards home-based diagnostic solutions opens new avenues for point-of-care molecular diagnostics.
- Development of Novel Biomarkers: Research into new biomarkers can lead to advanced diagnostic tests, creating new market opportunities.
- Government Initiatives: Government programs aimed at improving healthcare access and affordability can boost market growth.
- Increased Investment in R&D: Investment in research and development is expected to drive innovation and introduce advanced point-of-care diagnostics.
- Integration with Digital Health Platforms: Combining point-of-care diagnostics with digital health platforms offers new opportunities for data management and patient engagement.
Restraints
- High Initial Costs: The high cost of advanced point-of-care molecular diagnostic devices can be a barrier to market adoption.
- Regulatory Challenges: Stringent regulatory requirements can delay the approval and commercialization of new diagnostic products.
- Limited Reimbursement: Inadequate reimbursement policies can impact the affordability and accessibility of point-of-care molecular diagnostics.
- Technical Complexities: The complexity of molecular diagnostic technologies can limit their use in low-resource settings.
- Data Privacy Concerns: Issues related to data privacy and security can hinder the adoption of point-of-care diagnostics integrated with digital platforms.
- Competition from Traditional Diagnostics: Traditional diagnostic methods remain widely used and can compete with emerging point-of-care technologies.
- Lack of Skilled Personnel: The limited availability of trained healthcare professionals can affect the implementation and effective use of point-of-care molecular diagnostics.
Contact Us :
420 Lexington Avenue, Suite 300 New York City, NY 10170,
United States
Phone:+1 718 618 4351 (International),+91 78878 22626 (Asia)
Email: inquiry@market.us