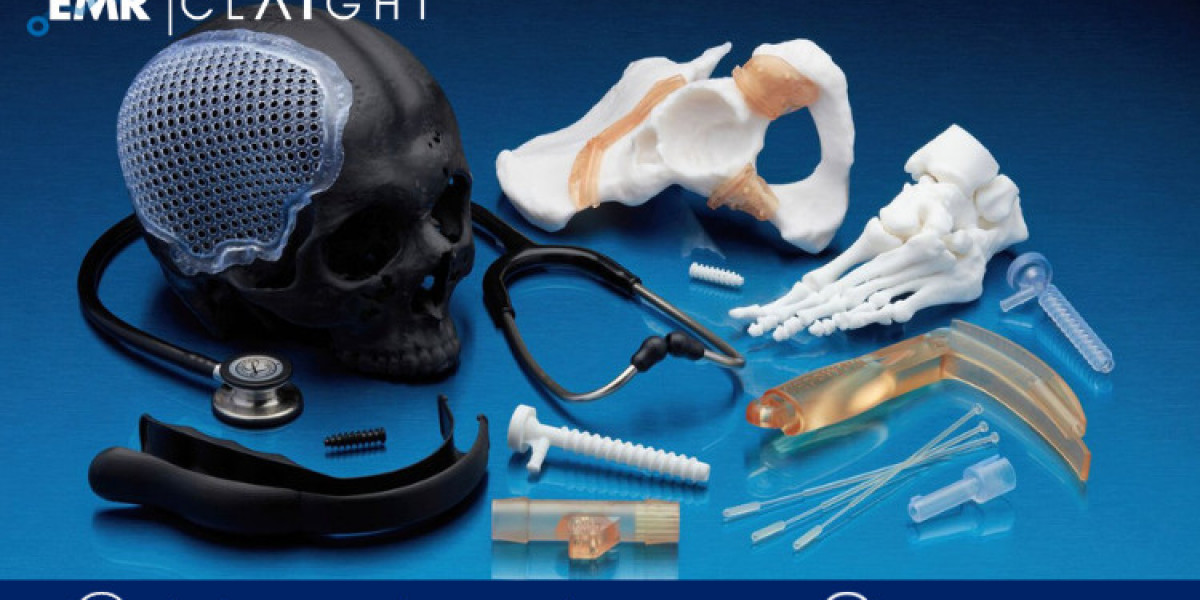
In recent years, 3D printing has emerged as a transformative technology across various industries, including healthcare. This blog explores the rapid evolution and promising future of the 3D Printing Medical Devices market, highlighting key trends, major players, regulatory influences, and future growth prospects.
Understanding 3D Printing in Medical Devices
Overview of 3D Printing Technology
3D printing, also known as additive manufacturing, involves creating three-dimensional objects layer by layer from digital models. In healthcare, this technology allows for the production of complex medical devices customized to individual patient needs, offering unprecedented design flexibility and efficiency.
Applications in Medical Device Manufacturing
The application of 3D printing in medical devices spans a wide range, including prosthetics, implants, surgical instruments, and anatomical models for surgical planning and training. This technology enables rapid prototyping, mass customization, and improved patient outcomes through personalized solutions.
Regulatory Landscape and Approvals
Navigating regulatory requirements, such as FDA approvals in the United States and CE marking in Europe, is crucial for market entry. Understanding these regulations ensures compliance and facilitates the adoption of 3D printed medical devices in clinical settings.
Market Size and Forecast
Current Market Landscape (2023)
As of 2023, the global 3D printing medical devices market was valued at USD 2.6 billion. This segment is driven by advancements in 3D printing technologies and increasing demand for personalized healthcare solutions.
Drivers of Market Growth
Factors fueling market growth include technological advancements in materials and printers, rising healthcare expenditure, and the shift towards patient-specific treatments. These drivers are projected to propel the market to reach USD 10.4 billion by 2032, with a CAGR of 16.8% during the forecast period (2024-2032).
Dynamics Shaping the Market
Key Drivers
- Technological Advancements: Continuous innovation in 3D printing materials and techniques enhances device performance and production efficiency.
- Personalized Medicine Demand: Growing preference for customized medical solutions tailored to individual patient anatomy and needs.
- Regulatory Support: Increasing regulatory approvals for 3D printed medical devices bolster market confidence and adoption rates.
Challenges
- Cost Barriers: High initial costs associated with 3D printing equipment and materials may limit market accessibility, particularly in developing regions.
- Regulatory Compliance: Stringent regulatory requirements and approval processes pose challenges to market entry and product commercialization.
Opportunities
- Emerging Market Expansion: Untapped opportunities in emerging economies for affordable and accessible healthcare solutions.
- Strategic Partnerships: Collaborations between technology providers, healthcare institutions, and regulatory bodies facilitate innovation and market penetration.
Competitive Landscape
Key Players Overview
Leading companies shaping the 3D printing medical devices market include established players like Stratasys Ltd., 3D Systems, Inc., and newer entrants focusing on specialized applications such as orthopedics and dental implants.
Strategic Initiatives
- Partnerships and Collaborations: Case studies such as EOS partnering with Tecomet, Precision ADM, and Orthopaedic Innovation Centre highlight collaborative efforts to enhance medical device manufacturing capabilities.
Technological Landscape
Types of 3D Printing Technologies
- Stereolithography (SLA): Utilizes photopolymerization to create high-resolution models and prototypes.
- Selective Laser Sintering (SLS): Uses laser energy to fuse powdered materials, suitable for complex geometries and durable parts.
- Fused Deposition Modeling (FDM): Deposits layers of thermoplastic materials, ideal for rapid prototyping and low-cost production.
Material Innovations
- Advancements in biocompatible materials, such as titanium alloys and bioresorbable polymers, expand the scope of applications for 3D printed medical devices.
Regulatory Environment
FDA and Global Regulations
Understanding regulatory frameworks governing medical device approvals ensures compliance with safety and efficacy standards, essential for market acceptance and patient trust.
Impact on Market Dynamics
Regulatory approvals streamline market entry and influence investment decisions, shaping market dynamics and innovation trajectories.
Market Segmentation
By Device Type
- Orthopedic Implants
- Dental Restorations
- Surgical Instruments
- Prosthetics and Orthotics
By Application
- Hospitals and Clinics
- Research Institutions
- Ambulatory Surgical Centers
By End-user
- Healthcare Providers
- Academic and Research Institutes
- Contract Manufacturing Organizations (CMOs)
Patent and Clinical Trials Analysis
Patent Trends
Analyzing patent filings provides insights into technological innovations and competitive strategies shaping the market landscape.
Clinical Trials Insights
Tracking clinical trials and research studies evaluates the safety, efficacy, and market readiness of 3D printed medical devices across therapeutic areas.
Investment and Funding Analysis
Venture Capital Trends
Investment in 3D printing startups and technology firms accelerates product development and market expansion initiatives.
Government Initiatives
Government funding and grants support research and development in advanced manufacturing technologies, fostering innovation and market competitiveness.
Challenges and Future Outlook
Key Challenges
Navigating cost constraints, regulatory hurdles, and market fragmentation pose challenges to market growth and adoption rates.
Future Trends and Innovations
Emerging trends in 3D printing, such as bioprinting and integrated healthcare ecosystems, offer transformative opportunities for personalized medicine and patient care.
Recommendations for Stakeholders
Strategic recommendations for stakeholders, including technology providers, healthcare providers, and policymakers, to capitalize on market opportunities and mitigate challenges.