Report Overview
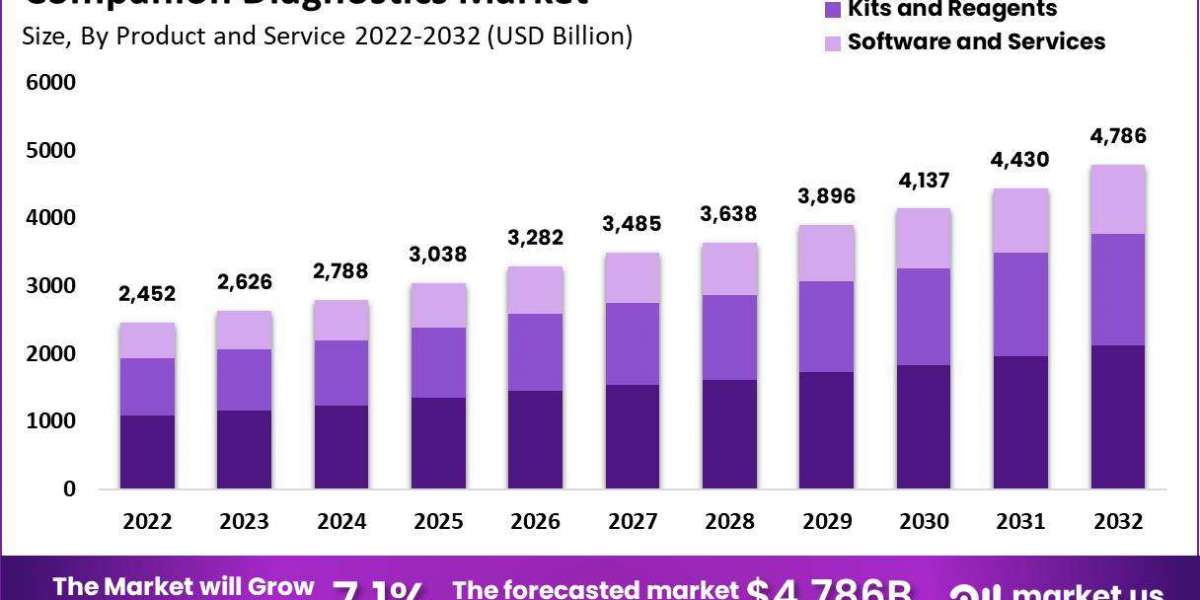
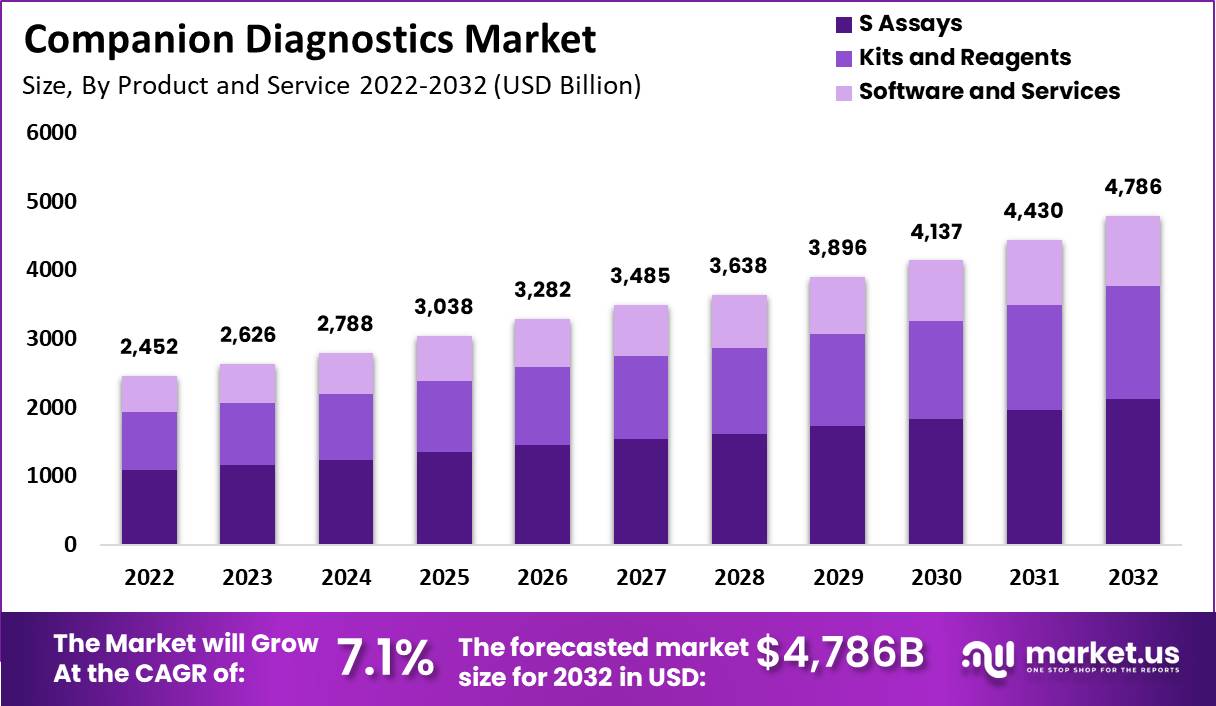
Companion Diagnostics Market size is expected to be worth around USD 4,786 Million by 2032 from USD 2,452 Million in 2022, growing at a CAGR of 7.1% during the forecast period from 2022 to 2032.
Key Takeaways
The global companion diagnostics market is expected to surpass USD 4,786 million by 2032, showing significant growth from its 2022 valuation of USD 2,452 million, growing at a CAGR of 7.1%.
North America led the companion diagnostics market in 2022, capturing a 43% revenue share, while Europe followed closely with a 25% share.
The Asia-Pacific region is poised for robust growth due to increasing cancer rates and enhancements in healthcare infrastructure.
Market growth is driven by factors such as heightened patient awareness, the prevalence of chronic diseases and allergies, and advancements in personalized medicine.
Among products and services, the assay segment has emerged as the top revenue generator in the companion diagnostics market.
Polymerase Chain Reaction (PCR) technology dominates the market and continues to expand rapidly.
Cancer care and technological innovations play pivotal roles in driving growth within the indication segment.
Pharmaceutical and biopharmaceutical companies hold the largest market share among end-users.
Key market influencers include the demand for cost-effective, high-quality care and adherence to regulatory standards.
Get a sample copy of the report to learn more https://market.us/report/companion-diagnostics-market/request-sample/
Key Market Segments
By Product and Service
- S Assays
- Kits and Reagents
- Software and Services
By Technology
- Polymerase Chain Reaction
- Next Generation Sequencing
- In Situ Hybridization
- Immunohistochemistry
By Indication
- Lung Cancer
- Breast Cancer
- Colorectal Cancer
- Leukemia
- Melanoma
By End-User
- Pharmaceutical and Biopharmaceutical Companies
- Reference Laboratories
- Contract Research Organizations
Key Regions
- North America (The US, Canada, Mexico)
- Western Europe (Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, Rest of Western Europe)
- Eastern Europe (Russia, Poland, The Czech Republic, Greece, Rest of Eastern Europe)
- APAC (China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, Rest of APAC)
- Latin America (Brazil, Colombia, Chile, Argentina, Costa Rica, Rest of Latin America)
- Middle East & Africa (Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, Rest of MEA)
Market Key Players
- Abbott (U.S.)
- Agilent Technologies Inc. (U.S.)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Guardant Health (U.S.)
- QIAGEN (Germany)
- Myriad Genetics, Inc. (U.S.)
- Illumina Inc. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- BIOMERIEUX (France)
- Myriad Genetics Inc. (U.S.)
- Other Key Players
If You Have Any Questions About This Report, Please Reach Out to Us https://market.us/report/companion-diagnostics-market/#inquiry
Drivers:
Increasing Demand for Personalized Medicine: Increase in the global usage of targeted therapies and biomarker-based targeting driving companion diagnostics.
Rising Prevalence of Chronic Diseases: Chronic diseases like cancer have high incidences, and thus proper diagnosis instruments will be needed for their identification.
Trends:
Advancements in Next-Generation Sequencing: Insertions in sequencing improve companion diagnostics’ effectiveness and capacity.
Shift towards Biomarker-Based Testing: Growing concentration on biomarkers as a means of identifying diseases and choosing the mode of treatment alters diagnostics.
Opportunities:
Expansion in Emerging Markets: There is largely unexplored potential in areas such as Asia-Pacific as healthcare improves there.
Integration with Telemedicine: Thus, growth in the utilization of telemedicine helps widen the availability and application of companion diagnostics.
Restraints:
Regulatory Challenges: Regulations both within the countries and standard global practices make it difficult to enter certain markets and release products.
High Development Costs: One limitation detected in the companion diagnostics industry is that the costs involved in research and technology innovation are expensive, especially for small companies.
Contact Us :
420 Lexington Avenue, Suite 300 New York City, NY 10170,
United States
Phone:+1 718 618 4351 (International),+91 78878 22626 (Asia)
Email: inquiry@market.us