Report Overview
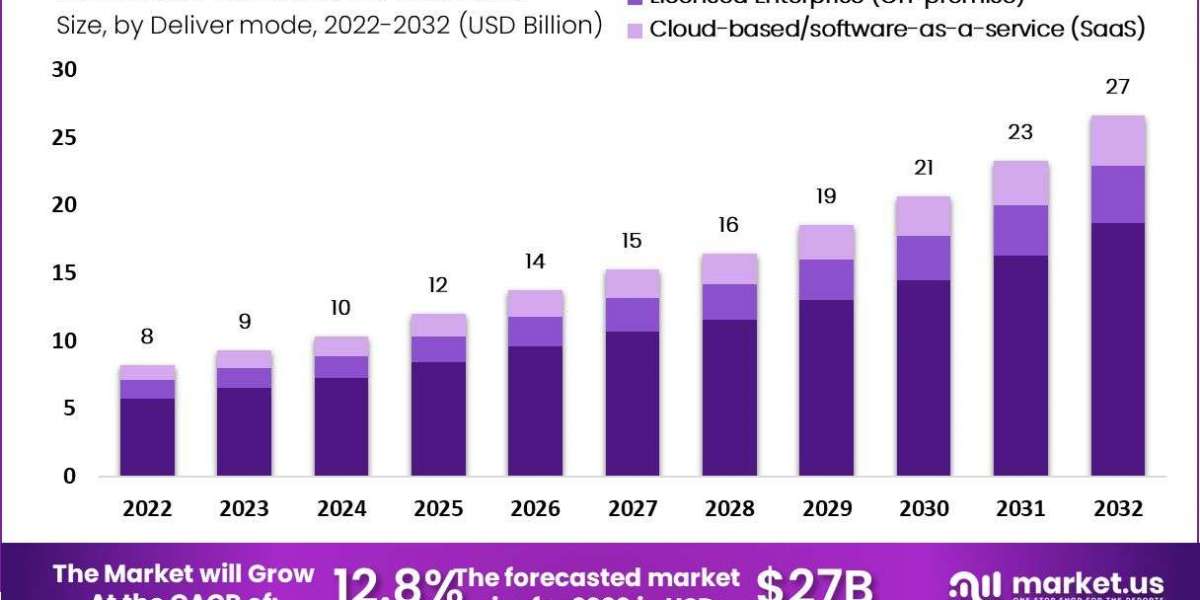
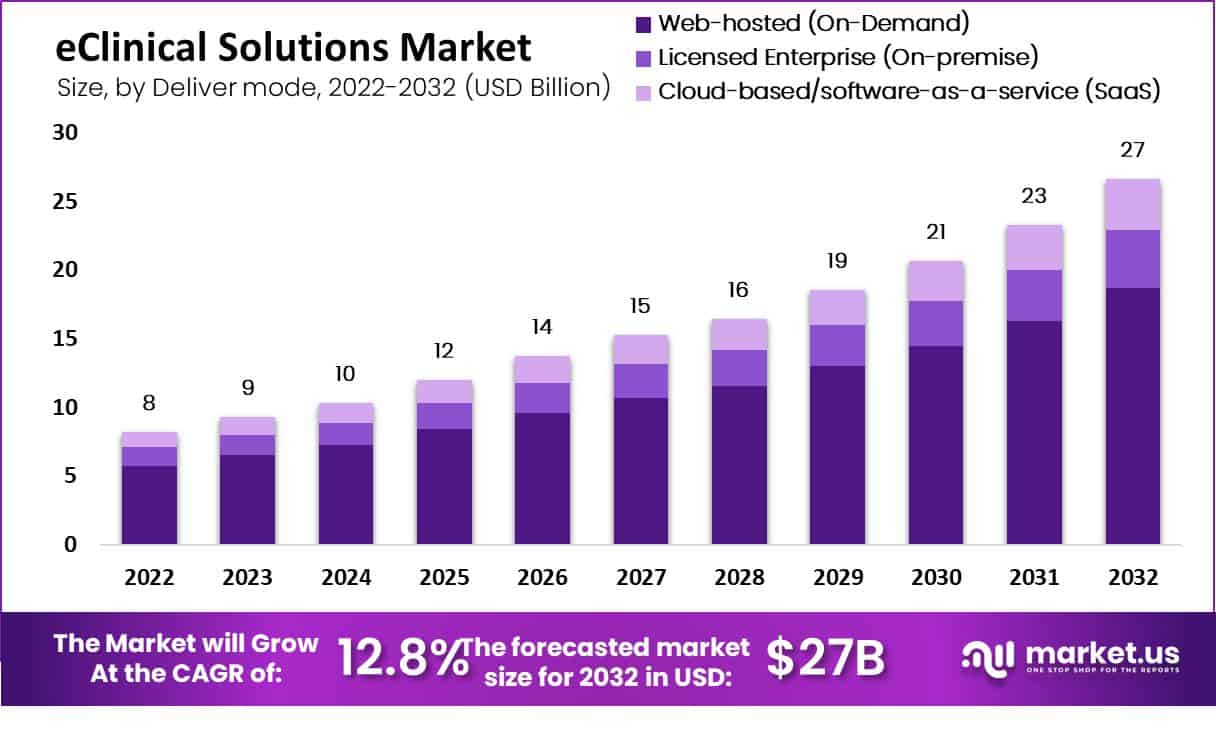
Global eClinical Solutions Market size is expected to be worth around USD 27 Billion by 2032 from USD 8 Billion in 2022, growing at a CAGR of 12.8% during the forecast period from 2022 to 2032
Key Takeaways:
The eClinical Solutions Market is set to expand significantly, reaching approximately $27 billion by 2032.
eCoA leads in product innovation, focusing on enhancing clinical data collection quality.
Web-hosted solutions dominated in 2022, while cloud-based solutions are poised for rapid growth.
Phase III trials are critical for evaluating drug effectiveness, with Phase I showing the highest growth potential.
CROs are the key end-users, projected to experience robust growth in the coming years.
 Get a sample copy of the report https://market.us/report/eclinical-solutions-market/request-sample/
Get a sample copy of the report https://market.us/report/eclinical-solutions-market/request-sample/
Key Market Segments
Product
- Electronic Data Capture (EDC)
- Clinical Data Management Systems (CDMS)
- Clinical Trial Management Systems (CTMS)
- Electronic Clinical Outcome Assessment (eCOA)
- Randomization and Trial Supply Management Solution (RTMS)
- Safety Solutions
- Analytics and Reporting Platforms
- Integration Platforms
- Electronic Trial Master File (eTMF)
Delivery Mode
- Web-hosted (On-Demand)
- Licensed Enterprise (On-premise)
- Cloud-based/software-as-a-service (SaaS)
Development Phase
- Phase I
- Phase II
- Phase III
- Phase IV
End-User
- Academic Institutes
- Medical Device Manufactures
- Hospitals
- CROs
- Pharmaceutical & Biotechnology Companies
Key Regions
- North America (The US, Canada, Mexico)
- Western Europe (Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, Rest of Western Europe)
- Eastern Europe (Russia, Poland, The Czech Republic, Greece, Rest of Eastern Europe)
- APAC (China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, Rest of APAC)
- Latin America (Brazil, Colombia, Chile, Argentina, Costa Rica, Rest of Latin America)
- Middle East & Africa (Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, Rest of MEA)
Market Key Players
- Oracle Corporation
- Medidata Solutions, Inc.
- Parexel International Corporation
- BioClinica, Inc.
- Signant Health
- Datatrak International, Inc.
- ERT
- eClinical Solutions, Inc.
- MaxisIT Inc.
- Bio-Optronics, Inc.
- Dassault Systemes
- IBM Watson Health
- Anju Life Sciences Software
- Merge Healthcare Incorporated
- OmniComm System
- Other Key Players
If You Have Any Questions About This Report, Please Reach Out to Us
https://market.us/report/eclinical-solutions-market/#inquiry
Drivers:
Rising adoption of electronic data capture systems and clinical trial management solutions that are efficient and qualitative, increase in chronic disease cases, expecting a need for clinical research; and technological advances in the accuracy and reliability of data.
Opportunities :
Geographical expansion in emerging markets, the addition of artificial intelligence and machine learning to the process of clinical trials, a growing number of virtual trials, and remote monitoring, including a hike in demand to provide real-time analytics and prediction.
Trends:
Increasingly moving towards cloud-based solutions.
Adoption of mobile and wearable technologies
Patient-centric approaches
Regulatory support to share EHRs across different healthcare organizations
Restraints :
High Implementation cost
Data privacy and security-related concerns
Inadequate number of skilled professionals in clinical trial management
Complexities in regulation and compliance
Contact Us :
420 Lexington Avenue, Suite 300 New York City, NY 10170,
United States
Phone:+1 718 618 4351 (International),+91 78878 22626 (Asia)
Email: inquiry@market.us