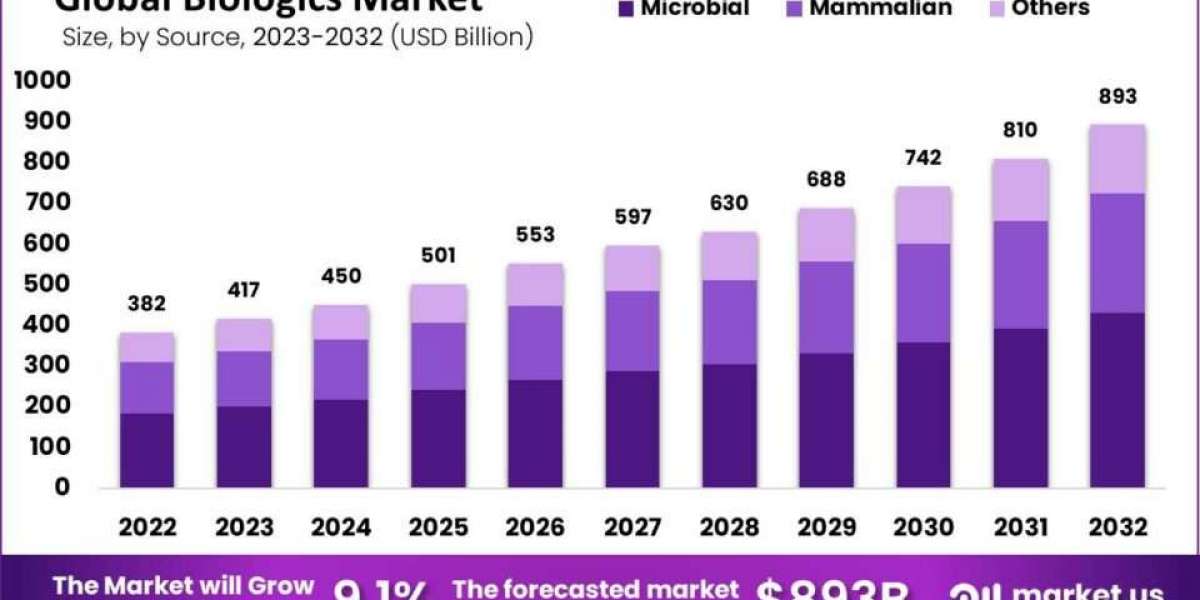
The global biologics market size is expected to be worth around USD 893.83 Billion by 2032 from USD 382.81 Billion in 2022, growing at a CAGR of 9.10% during the forecast period from 2022 to 2032.
Get a sample copy of the report to know more https://market.us/report/biologics-market/request-sample/
Key Market Segments
Based On Source
- Microbial
- Mammalian
- Others
Based on Product
- MABs
- Vaccines
- Hormones
- Therapeutic Enzymes
- Recombinant Proteins
- Antisense, RNAi & Molecular Therapy
- Blood Factors and Anticoagulants
- Allergenic extracts
- Human Cells and Tissues
- Proteins
- Gene Therapies
- Cellular Therapies
- Others
Based on Disease
- Oncology
- Immunological Disorders
- Cardiovascular Disorders
- Hematological Disorders
- Others
Based on Manufacturing
- Outsourced
- In-house
Based on the Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Key Regions
- North America (The US, Canada, Mexico)
- Western Europe (Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, Rest of Western Europe)
- Eastern Europe (Russia, Poland, The Czech Republic, Greece, Rest of Eastern Europe)
- APAC (China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, Rest of APAC)
- Latin America (Brazil, Colombia, Chile, Argentina, Costa Rica, Rest of Latin America)
- Middle East & Africa (Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, Rest of MEA)
Market Key Players
- Amgen Inc.
- F. Hoffmann-La Roche Ltd
- AbbVie Inc.
- AstraZeneca plc
- Merck KGaA
- Sanofi
- GlaxoSmithKline plc
- Johnson & Johnson
- Pfizer Inc.
- Novartis AG
- Eli Lilly and Company
If You Have Any Questions About This Report, Please Reach Out to Us @ https://market.us/report/enteral-feeding-devices-market/#inquiry
Drivers
Rising Prevalence of Chronic Diseases
The increasing incidence of chronic diseases like cancer, diabetes, and autoimmune disorders drives the demand for biologics. Biologics offer targeted treatment options, improving patient outcomes and reducing side effects.Advancements in Biotechnology
Technological advancements in biotechnology have enhanced the development of biologics. Innovations such as recombinant DNA technology and gene therapy are propelling the market forward.Growing Approval and Adoption
The increasing number of FDA approvals for biologics is a significant market driver. This trend is supported by the high adoption rates among healthcare professionals and patients.Government Support and Funding
Governments worldwide are offering support through funding and favorable policies. These initiatives encourage research and development in biologics, boosting market growth.
Trends
Shift Towards Personalized Medicine
Personalized medicine is gaining traction, with biologics playing a crucial role. Biologics are tailored to individual patient profiles, enhancing treatment efficacy and minimizing adverse effects.Rise of Biosimilars
The emergence of biosimilars is a notable trend in the biologics market. Biosimilars offer a cost-effective alternative to original biologics, increasing accessibility to treatments.Expansion in Emerging Markets
Emerging markets in Asia-Pacific and Latin America are witnessing significant growth. Increased healthcare spending and improved infrastructure are driving biologics demand in these regions.Integration of Artificial Intelligence (AI)
AI is increasingly being used in biologics research and development. AI helps in predicting drug responses, optimizing clinical trials, and accelerating the drug discovery process.
Opportunities
Unmet Medical Needs
There are numerous unmet medical needs in various therapeutic areas. Biologics present an opportunity to address these needs, particularly in oncology, immunology, and rare diseases.Partnerships and Collaborations
Strategic partnerships between biotech firms and pharmaceutical companies offer growth opportunities. Collaborations accelerate the development of new biologics and expand market reach.Regulatory Pathways for Accelerated Approvals
Regulatory agencies are offering accelerated approval pathways for biologics targeting life-threatening conditions. This provides an opportunity for faster market entry and early revenue generation.Expanding Use in Non-Traditional Areas
The application of biologics is expanding beyond traditional areas. New uses in dermatology, ophthalmology, and neurology present untapped opportunities for market growth.
Restraints
High Development Costs
The development of biologics is expensive, posing a significant market restraint. High costs impact the pricing and accessibility of biologics, limiting market expansion.Stringent Regulatory Requirements
Biologics face stringent regulatory requirements, which can delay approvals and market entry. Complex regulatory processes add to the development timeline and costs.Manufacturing Challenges
Biologics manufacturing is complex and requires specialized facilities and expertise. Manufacturing challenges, including scale-up issues and quality control, can hinder market growth.Patent Expiry and Competition
The expiration of biologics patents leads to increased competition from biosimilars. Patent expiries can significantly impact revenue streams for original biological manufacturers.
Contact Us :
420 Lexington Avenue, Suite 300 New York City, NY 10170,
United States
Phone:+1 718 618 4351 (International),+91 78878 22626 (Asia)
Email: inquiry@market.us