United States Influenza Diagnostics Market Overview
Base Year: 2023
Historical Years: 2018-2023
Forecast Years: 2024-2032
Market Growth Rate: 6.30% (2024-2032)
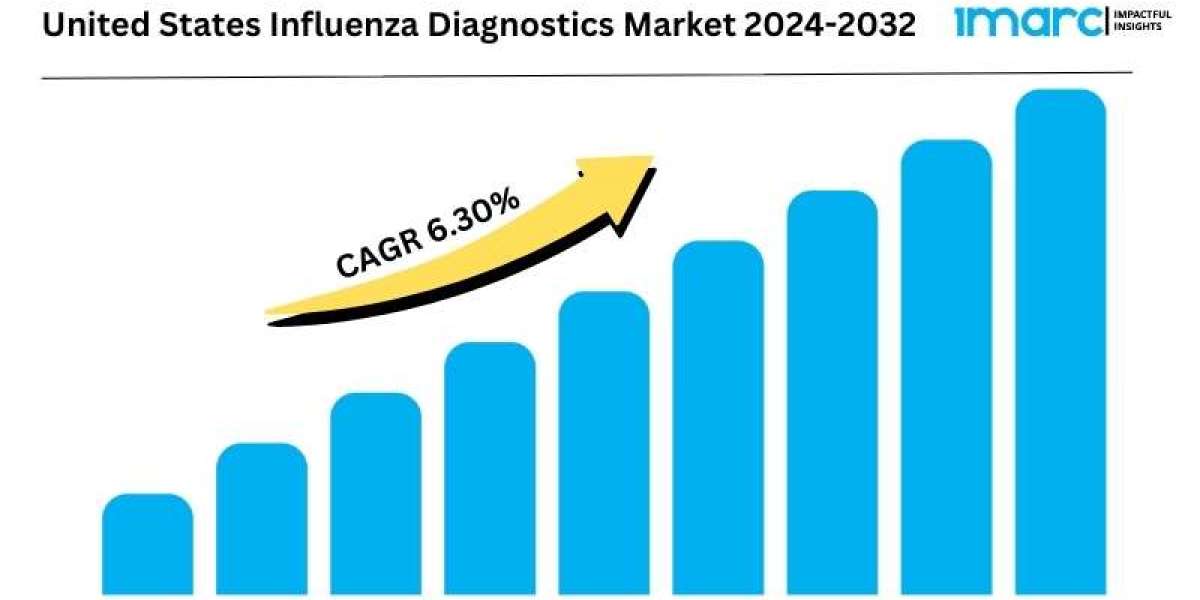
The U.S. influenza diagnostics market size is expanding rapidly, driven by increasing demand for accurate and timely diagnosis of influenza infections. This growth is fueled by advancements in diagnostic technologies and the growing focus on public health and pandemic preparedness. According to the latest report by IMARC Group, the market is projected to grow at a CAGR of 6.30% from 2024 to 2032.
United States Influenza Diagnostics Industry Trends and Drivers:
The United States influenza diagnostics market is witnessing key trends shaped by advancements in diagnostic technologies and the growing need for rapid, accurate testing. Point-of-care (POC) diagnostics are gaining prominence due to their ability to deliver immediate results, facilitating timely treatment decisions and reducing the spread of infection.
Technological improvements, such as molecular diagnostics and digital flu tests, are becoming more widely adopted, offering higher sensitivity and specificity compared to traditional methods. The market is also seeing an increasing shift toward multiplex testing, which allows simultaneous detection of multiple respiratory viruses, including influenza, in a single test. This trend is particularly relevant in healthcare settings where quick differentiation between similar viral infections is essential.
Additionally, the integration of artificial intelligence and machine learning is enhancing diagnostic accuracy and operational efficiency. The ongoing emphasis on preventive healthcare and early detection is further contributing to the rise in demand for influenza diagnostics in the United States.
The growth of the United States influenza diagnostics market is being driven by the increasing prevalence of influenza cases, especially during seasonal outbreaks, is a key driver as healthcare providers and public health agencies aim to minimize the disease's impact through early diagnosis and treatment. Government initiatives promoting influenza vaccination and awareness campaigns are also supporting the market, as more people seek testing to confirm influenza before proceeding with treatment.
Additionally, the demand for advanced diagnostics has surged in recent years due to the COVID-19 pandemic, which highlighted the importance of accurate and timely detection of respiratory infections. This has led to a greater focus on improving diagnostic infrastructure and capabilities in the United States market. The growing trend of home-based testing is another significant driver, with consumers seeking convenient and accessible options for flu diagnostics.
Manufacturers are responding by developing user-friendly kits that allow individuals to perform tests at home with minimal effort. Lastly, the rise in healthcare spending, coupled with the expansion of healthcare facilities and services across the country, is further propelling the growth of the influenza diagnostics market. These factors collectively contribute to a robust market outlook for the coming years.
United States Influenza Diagnostics Industry Segmentation:
The report has segmented the market into the following categories:
Product Insights:
- Test Kit and Reagents
- Instruments
- Others
Test Type Insights:
- Molecular Diagnostic Tests
- Polymerase Chain Reaction
- Isothermal Nucleic Acid Amplification Tests
- Others
- Traditional Diagnostic Tests
- Rapid Influenza Diagnostic Tests
- Viral Culture Tests
- Direct Fluorescent Antibody Test
- Serological Tests
Type of Flu Insights:
- Type A Flu
- Type B Flu
- Type C Flu
End User Insights:
- Hospitals
- Diagnostic Laboratories
- Others
Regional Insights:
- Northeast
- Midwest
- South
- West
Competitive Landscape:
The competitive landscape of the industry has also been examined along with the profiles of the key players.
Key highlights of the Report:
- Market Performance (2018-2023)
- Market Outlook (2024-2032)
- COVID-19 Impact on the Market
- Porter’s Five Forces Analysis
- Strategic Recommendations
- Historical, Current and Future Market Trends
- Market Drivers and Success Factors
- SWOT Analysis
- Structure of the Market
- Value Chain Analysis
- Comprehensive Mapping of the Competitive Landscape
Note: If you need specific information that is not currently within the scope of the report, we can provide it to you as a part of the customization.
Ask analyst for your customized sample: https://www.imarcgroup.com/request?type=report&id=20572&flag=F
| U.S. Orthobiologics Market size is projected to exhibit a growth rate (CAGR) of 3.5% during 2024-2032. |
| U.S. Neurological Biomarkers Market size is projected to exhibit a growth rate (CAGR) of 4.2% during 2024-2032. |
| U.S. Pasta Market size is projected to exhibit a growth rate (CAGR) of 8.25% during 2024-2032. |
| U.S. Industrial Iot Market size is projected to exhibit a growth rate (CAGR) of 16.4% during 2024-2032. |
| U.S. Lead Acid Battery Market size is projected to exhibit a growth rate (CAGR) of 3.21% during 2024-2032. |
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145