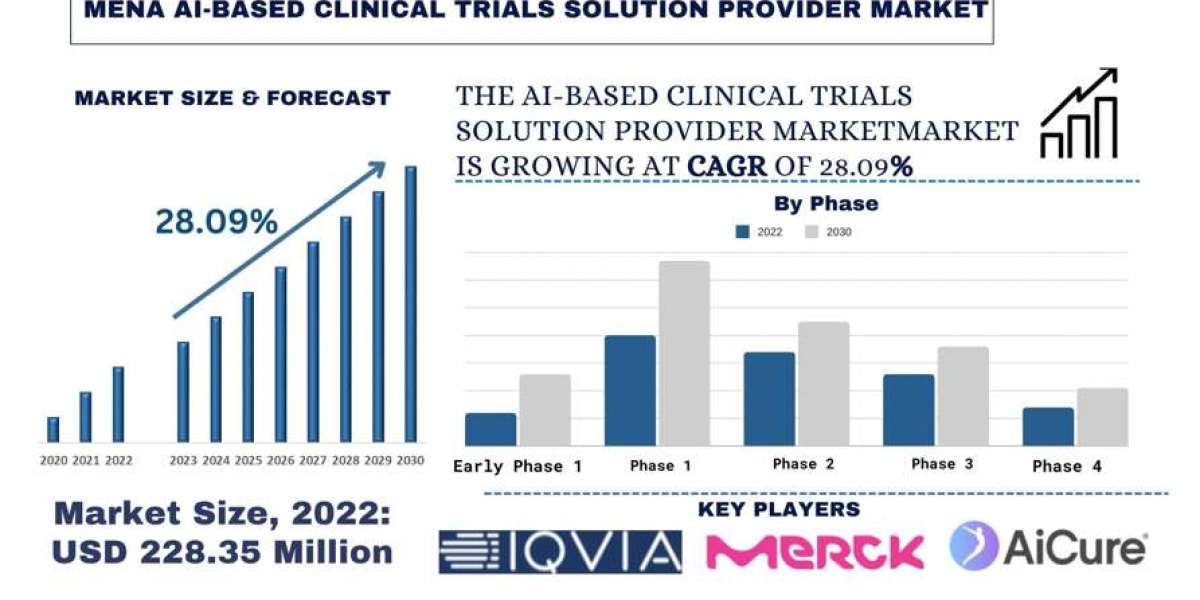
According to a new report by Univdatos Market Insights, the MENA AI-Based Clinical Trials Solution Provider Market was valued at USD 228.35 million in the year 2022 and is expected to grow at a strong CAGR of around 28.09% during the forecast period. With the increasing need for faster and more accurate clinical trials, AI-based solutions are in demand to streamline processes. Adding to this, continuous advancements in AI technologies like machine learning and natural language processing are enhancing the capabilities of clinical trial solutions. Moreover, rising chronic diseases and the need for personalized medicine solutions are driving pharmaceutical companies and research institutions to embrace AI technologies for more efficient and targeted clinical trials. This growing collaborations between academic institutions, pharmaceutical companies, and AI startups are driving innovation. For instance, on 20 September 2023, Merck partnered with BenevolentAI and Exscientia to drive accelerated drug discovery with a higher probability of success. Access to end-to-end AI platform capabilities to generate novel development candidates in oncology, neurology, and immunology.
Request To Download Sample of This Strategic Report- https://univdatos.com/get-a-free-sample-form-php/?product_id=57596&utm_source=LinkSJ&utm_medium=Snehal&utm_campaign=Snehal&utm_id=snehal
The report suggests that AI-Based Clinical Trials Solution Provider resources in the MENA region had a significant impact on the AI-Based Clinical Trials Solution Provider industry in the MENA region. Some of how this impact has been felt include:
For instance, on October 23, 2023, UAE Ministry of Finance launched digital transformation initiatives using metaverse and AI solutions.
For instance, in August 2023, Israel company QuantHealth captured USD 15 million in series A funding to expand the reach of AI-driven trial design.
As per the latest research of DHCC’s estimates that the combined digital health market in Saudi Arabia and For instance, on May 10, 2021, The Saudi Data and Artificial Intelligence Authority (SDAIA), represented by its innovative arm, the Center for Artificial Intelligence signed a memorandum of understanding (MOU) with IQVIA exploring opportunities of mutual interest to advance innovation in the field of healthcare data in the Kingdom.
Apart from this, in recent years, several governments in the MENA and North Africa have implemented policies and provided funding support to enhance the growth of the AI-Based Clinical Trials Solution Provider industry. Some examples include:
Regional countries accept all GCC-DR decisions. The Applicant only needs to submit the approval certificate to the GCC-DR and price certificates to the national competent authorities for the national approval process.
Egyptian Drug Authority (EDA) has replaced NORCB and NODCAR through the presidential decree presidential decree no (151) of (2019). EDA is engaged in a close collaborative effort with other regulatory authorities where there is strong coordination between all bodies responsible for enforcing laws and regulations relating to biological products, pharmaceutical products, medical devices, and herbal medicines to ensure that the principles of the GCP are applied.
Ask for Report Customization - https://univdatos.com/get-a-free-sample-form-php/?product_id=57596&utm_source=LinkSJ&utm_medium=Snehal&utm_campaign=Snehal&utm_id=snehal
Browse Related Reports:
· Opioid Use Disorder (OUD) Treatment Market
Conclusion
In conclusion, the MENA region is at the forefront of harnessing AI's transformative power in clinical trials, with AI-based Clinical Trials Solution Providers driving innovation, collaboration, and efficiency across the healthcare ecosystem. As these trends continue to unfold, stakeholders must navigate the evolving landscape adeptly, embracing ethical AI practices, fostering partnerships, and leveraging AI's full potential to usher in a new era of precision medicine and patient-centered care in the MENA region and beyond.