IMARC Group has recently released a new research study titled “United States Biosimilar Market Size, Share, Trends and Forecast by Molecule, Manufacturing Type, Indication, and Region, 2025-2033”, offers a detailed analysis of the market drivers, segmentation, growth opportunities, trends and competitive landscape to understand the current and future market scenarios.
United States Biosimilar Market Overview
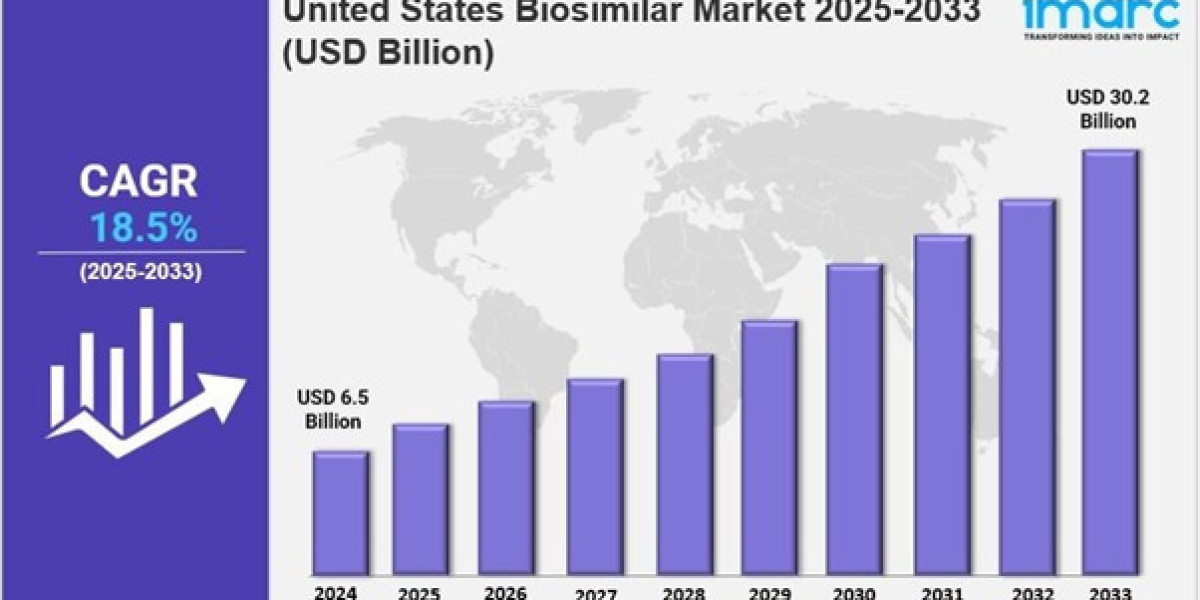
The United States biosimilar market size was valued at USD 6.5 Billion in 2024. Looking forward, IMARC Group estimates the market to reach USD 30.2 Billion by 2033, exhibiting a CAGR of 18.5% from 2025-2033.
Market Size and Growth
Base Year: 2024
Forecast Years: 2025-2033
Historical Years: 2019-2024
Market Size in 2024: USD 6.5 Billion
Market Forecast in 2033: USD 30.2 Billion
Market Growth Rate (2025-2033): 18.5%
Request for a sample copy of the report: https://www.imarcgroup.com/united-states-biosimilar-market/requestsample
Key Market Highlights:
✔️ Expanding market driven by increased healthcare costs and a push for affordable treatment options.
✔️ Growing acceptance and adoption of biosimilars among healthcare professionals and patients.
✔️ Strong regulatory support enhancing the approval process for biosimilar products.
✔️ Rising prevalence of chronic diseases boosting the demand for biologic therapies.
✔️ Increased focus on innovative drug development fostering competition in the biosimilars sector.
United States Biosimilar Market Trends and Drivers
Rise in Acceptance of Biosimilars
A notable trend influencing the United States Biosimilar Market is the increasing acceptance of biosimilars among healthcare providers and patients. As more biosimilars receive regulatory approval, healthcare professionals are becoming more familiar with their efficacy and safety profiles. This growing trust is essential for promoting the use of biosimilars as cost-effective alternatives to reference biologics. By 2025, the market is expected to see a significant rise in biosimilar adoption, driven by educational initiatives and positive clinical outcomes that reinforce their role in treatment protocols.
Expansion of Therapeutic Areas
Another important trend is the expansion of biosimilars into a broader range of therapeutic areas. Initially focused on oncology and autoimmune diseases, the United States Biosimilar Market size is projected to grow as biosimilars are developed for various conditions, including diabetes and cardiovascular diseases. This diversification not only enhances treatment options for patients but also increases market competition, which can lead to lower prices. By 2025, the introduction of new biosimilars across multiple therapeutic categories will likely contribute to a more dynamic market landscape, benefiting both patients and healthcare systems.
Competitive Pricing Strategies
The United States Biosimilar Market share is also being shaped by competitive pricing strategies employed by biosimilar manufacturers. As the market matures, companies are focusing on pricing models that offer significant savings compared to their reference biologics. This strategy is crucial in encouraging healthcare providers to prescribe biosimilars, especially in a cost-sensitive environment. By 2025, the emphasis on affordability will likely enhance the market's growth, making biosimilars a more attractive option for payers and patients alike.
Regulatory Support and Guidance
Finally, regulatory support and streamlined approval processes are playing a critical role in the United States Biosimilar Market growth. The U.S. Food and Drug Administration (FDA) has been actively working to create a favorable environment for biosimilar development through clear guidelines and expedited review pathways. This support is vital for fostering innovation and encouraging investment in biosimilars. By 2025, continued regulatory advancements will likely facilitate the entry of new biosimilars into the market, further solidifying their presence as a key component of modern therapeutics.
Speak to An Analyst: https://www.imarcgroup.com/request?type=report&id=11073&flag=C
United States Biosimilar Market Segmentation:
The market report segments the market based on product type, distribution channel, and region:
Analysis by Molecule:
- Infliximab
- Insulin Glargine
- Epoetin Alfa
- Etanercept
- Filgrastim
- Somatropin
- Rituximab
- Follitropin Alfa
- Adalimumab
- Pegfilgrastim
- Trastuzumab
- Bevacizumab
- Others
Analysis by Manufacturing Type:
- In-house Manufacturing
- Contract Manufacturing
Analysis by Indication:
- Auto-Immune Diseases
- Blood Disorder
- Diabetes
- Oncology
- Growth Deficiency
- Female Infertility
- Others
Regional Analysis:
- Northeast
- Midwest
- South
- West
Competitive Landscape:
The market research report offers an in-depth analysis of the competitive landscape, covering market structure, key player positioning, top winning strategies, a competitive dashboard, and a company evaluation quadrant. Additionally, detailed profiles of all major companies are included.
Key Highlights of the Report
- Market Performance (2019-2024)
- Market Outlook (2025-2033)
- COVID-19 Impact on the Market
- Porter’s Five Forces Analysis
- Strategic Recommendations
- Historical, Current and Future Market Trends
- Market Drivers and Success Factors
- SWOT Analysis
- Structure of the Market
- Value Chain Analysis
- Comprehensive Mapping of the Competitive Landscape
About Us:
IMARC Group is a leading market research company that offers management strategy and market research worldwide. We partner with clients in all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses.
IMARC’s information products include major market, scientific, economic and technological developments for business leaders in pharmaceutical, industrial, and high technology organizations. Market forecasts and industry analysis for biotechnology, advanced materials, pharmaceuticals, food and beverage, travel and tourism, nanotechnology and novel processing methods are at the top of the company’s expertise.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145